There’s no worse feeling than watching a loved one fight bravely through a cancer diagnosis and battle through treatments while hoping for a miracle or a little more time. Suddenly, your doctor tells you that there’s nothing more they can do, and that palliative care is the only option left. The crushing feeling of hopelessness sets in, and it’s a moment that sits with you forever. Sadly, this is an experience shared by far too many families around the world. I know this all too well because I was there with my own family. It’s a moment etched in my memory, a moment that has fueled my desire to make a difference.
This is why I am excited to introduce our new company called Pathos, which focuses on bringing the benefits of AI to drug development, as there are few spaces in the world more in need of technology advances. Cancer is a collection of hundreds of sub-diseases and can be best described by biological pathway activity accounting for the pathophysiology of patients. To best treat these diseases, we need to stratify patient populations based on pathways for new therapeutic options and harness the power of AI to re-imagine each step of the drug development process.
Approach to Drug Development for Precision Medicine
At Pathos, we believe that the standard drug development approach for precision medicine needs a full rebuild. With a success rate of only 16% in oncology1, incremental improvements over the past decade are not enough for patients in need. Instead of tweaks or enhancements, we’re focused on re-engineering the entire process of drug development for precision medicine, starting with positioning clinical stage assets for successful phase II results. With this approach, we intend to partner with biopharmaceutical companies, not compete with them.
Phase I trials are traditionally testing for safety and tolerability of a drug, resulting in many all-comers trial designs that generate underpowered signals for patient selection strategies for a future phase II. Designing these patient selection strategies requires accessing rich patient datasets that can address systems biology questions, so we no longer need to rely on synthetic datasets generated in a lab or outdated publications to evaluate biomarker-driven opportunities.
Thankfully the industry has changed dramatically since the human genome project over 20 years ago. I’ve seen this first-hand while building and scaling Tempus over the past 8 years. With the latest advancements in generating real-world multimodal data, computational biology, and machine learning techniques (like shown in the image below) we can now access thousands of patient datasets or hundreds of petabytes of real-world data like never before.
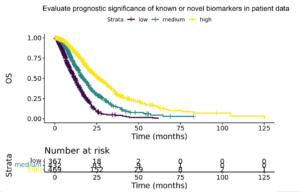
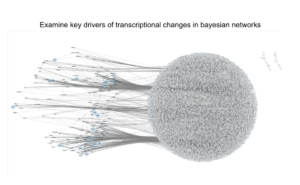
AI-driven Future Where No Patient is Left Behind
With real-world multimodal datasets at our fingertips, we’ve been building models of disease and uncovering patient selection strategies that target specific subpopulations based on biological pathways rather than tumor types classified by their organ of origin. However, building hypotheses in-silico isn’t enough. We’ve also acquired and integrated Known Medicine, a company that builds patient-specific 3D cell cultures & uses AI approaches to prospectively predict drug responses in patients. In doing so, we’re building a future where it is cost-effective to develop therapeutics for smaller populations to ensure no patient gets left behind. Developing drugs for these smaller populations doesn’t always make economic sense for companies but we’re fixated on solving the hard challenges to ensure these drugs can get to the patients that need them most.
Our platform is already being leveraged as we’ve licensed our first clinical-stage asset, P-300 (formerly known as FT-7051 from Novo Nordisk). P-300 was designed to inhibit CBP/p300 in advanced metastatic castrate resistant prostate cancer. It has recently completed an initial phase I trial. Additional details about the agreement with Novo Nordisk can be found here.
Off to the Races
We’ve been fortunate to raise $40 million dollars with like-minded investors to change the status quo and apply AI approaches throughout the drug development continuum to double, or even triple, the current benchmarks of probability of success in drug development.
With an experienced team of ex-founders, drug developers, and scientists, we are simultaneously building our computational platform with reverse translational approaches and developing life-altering therapeutics for patients at unparalleled speed. We’ve got big aspirations and we are dedicated to attracting the brightest minds and the boldest hearts to join us on this mission, propelling us towards a brighter future for cancer patients everywhere.
For now, we’re heads down building the future of drug development for precision medicine. We want our results to speak for themselves and will share details as we make meaningful progress toward improving the lives of cancer patients. More to come soon!

